What Does Capillary Action Do For Plants And Animals
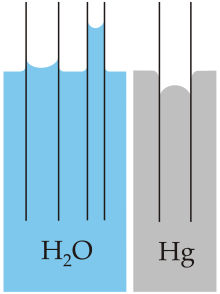
Capillary action of water (polar) compared to mercury (not-polar), in each case with respect to a polar surface such as glass (≡Si–OH)
Capillary action (sometimes chosen capillarity, capillary motility, capillary effect, or wicking) is the process of a liquid flowing in a narrow space without the assistance of, or even in opposition to, any external forces like gravity. The issue can be seen in the cartoon up of liquids betwixt the hairs of a paint-brush, in a sparse tube, in porous materials such equally paper and plaster, in some non-porous materials such equally sand and liquefied carbon fiber, or in a biological cell. It occurs because of intermolecular forces betwixt the liquid and surrounding solid surfaces. If the diameter of the tube is sufficiently small, and so the combination of surface tension (which is caused by cohesion within the liquid) and adhesive forces betwixt the liquid and container wall human activity to propel the liquid.[ane]
Etymology [edit]
Capillary comes from the Latin word capillaris, meaning "of or resembling hair." The meaning stems from the tiny, hairlike diameter of a capillary. While capillary is usually used as a substantive, the word besides is used every bit an describing word, as in "capillary action," in which a liquid is moved forth — even upward, confronting gravity — as the liquid is attracted to the internal surface of the capillaries.
Transpiration [edit]
Mass period of liquid h2o from the roots to the leaves is driven in office by capillary action, but primarily driven by water potential differences. If the water potential in the ambient air is lower than the water potential in the leaf airspace of the stomatal pore, h2o vapor volition travel down the slope and movement from the leaf airspace to the atmosphere. This movement lowers the h2o potential in the leaf airspace and causes evaporation of liquid h2o from the mesophyll jail cell walls. This evaporation increases the tension on the water menisci in the cell walls and decrease their radius and thus the tension that is exerted on the water in the cells. Because of the cohesive properties of h2o, the tension travels through the leaf cells to the leafage and stem xylem where a momentary negative pressure is created as h2o is pulled upwardly the xylem from the roots.[2] As evaporation occurs at the leaf surface, the properties of adhesion and cohesion work in tandem to pull water molecules from the roots, through xylem tissue, and out of the institute through stomata.[3] In taller plants and trees, the force of gravity tin can just be overcome by the decrease in hydrostatic (water) pressure level in the upper parts of the plants due to the improvidence of water out of stomata into the temper. Water is captivated at the roots by osmosis, and whatsoever dissolved mineral nutrients travel with it through the xylem.
History [edit]
The first recorded ascertainment of capillary action was by Leonardo da Vinci.[4] [5] A onetime educatee of Galileo, Niccolò Aggiunti, was said to have investigated capillary action.[half dozen] In 1660, capillary action was still a novelty to the Irish pharmacist Robert Boyle, when he reported that "some inquisitive French Men" had observed that when a capillary tube was dipped into water, the water would ascend to "some summit in the Pipage". Boyle and then reported an experiment in which he dipped a capillary tube into red wine and then subjected the tube to a partial vacuum. He institute that the vacuum had no observable influence on the meridian of the liquid in the capillary, so the beliefs of liquids in capillary tubes was due to some phenomenon different from that which governed mercury barometers.[seven]
Others soon followed Boyle's lead.[viii] Some (east.g., Honoré Fabri,[9] Jacob Bernoulli[10]) thought that liquids rose in capillaries because air could non enter capillaries as easily equally liquids, so the air pressure was lower inside capillaries. Others (e.thousand., Isaac Vossius,[11] Giovanni Alfonso Borelli,[12] Louis Carré,[13] Francis Hauksbee,[14] Josia Weitbrecht[15]) idea that the particles of liquid were attracted to each other and to the walls of the capillary.
Although experimental studies continued during the 18th century,[sixteen] a successful quantitative treatment of capillary activity[17] was not attained until 1805 by two investigators: Thomas Young of the United Kingdom[eighteen] and Pierre-Simon Laplace of French republic.[xix] They derived the Young–Laplace equation of capillary action. By 1830, the German mathematician Carl Friedrich Gauss had determined the boundary weather condition governing capillary action (i.e., the conditions at the liquid-solid interface).[20] In 1871, the British physicist William Thomson, 1st Baron Kelvin determined the effect of the meniscus on a liquid's vapor force per unit area—a relation known as the Kelvin equation.[21] German language physicist Franz Ernst Neumann (1798–1895) later on determined the interaction between two immiscible liquids.[22]
Albert Einstein's first paper, which was submitted to Annalen der Physik in 1900, was on capillarity.[23] [24]
Phenomena and physics [edit]

Capillary penetration in porous media shares its dynamic mechanism with flow in hollow tubes, as both processes are resisted past pasty forces.[25] Consequently, a common apparatus used to demonstrate the miracle is the capillary tube. When the lower cease of a glass tube is placed in a liquid, such as water, a concave meniscus forms. Adhesion occurs betwixt the fluid and the solid inner wall pulling the liquid column along until at that place is a sufficient mass of liquid for gravitational forces to overcome these intermolecular forces. The contact length (around the edge) between the top of the liquid cavalcade and the tube is proportional to the radius of the tube, while the weight of the liquid column is proportional to the square of the tube'south radius. So, a narrow tube volition depict a liquid column forth farther than a wider tube will, given that the inner water molecules cohere sufficiently to the outer ones.
Examples [edit]
In the built environment, evaporation limited capillary penetration is responsible for the phenomenon of rising clammy in concrete and masonry, while in industry and diagnostic medicine this phenomenon is increasingly being harnessed in the field of paper-based microfluidics.[25]

Consequence of placing a porous brick in a shallow tray of water

Moderate rising clammy on an internal wall
In physiology, capillary action is essential for the drainage of continuously produced tear fluid from the eye. Two canaliculi of tiny diameter are nowadays in the inner corner of the eyelid, also called the lacrimal ducts; their openings can be seen with the naked eye inside the lacrymal sacs when the eyelids are everted.
Wicking is the absorption of a liquid by a material in the way of a candle wick. Paper towels blot liquid through capillary action, allowing a fluid to be transferred from a surface to the towel. The minor pores of a sponge act equally minor capillaries, causing it to absorb a large amount of fluid. Some textile fabrics are said to apply capillary action to "wick" sweat away from the skin. These are oftentimes referred to as wicking fabrics, afterwards the capillary properties of candle and lamp wicks.
Capillary activeness is observed in thin layer chromatography, in which a solvent moves vertically upwards a plate via capillary action. In this case the pores are gaps between very small particles.
Capillary activity draws ink to the tips of fountain pen nibs from a reservoir or cartridge inside the pen.
With some pairs of materials, such as mercury and glass, the intermolecular forces within the liquid exceed those between the solid and the liquid, and so a convex meniscus forms and capillary activeness works in reverse.
In hydrology, capillary action describes the attraction of water molecules to soil particles. Capillary action is responsible for moving groundwater from wet areas of the soil to dry areas. Differences in soil potential ( ) bulldoze capillary action in soil.
A practical awarding of capillary activity is the capillary action siphon. Instead of utilizing a hollow tube (equally in most siphons), this device consists of a length of cord made of a fibrous textile (cotton string or string works well). After saturating the cord with water, one (weighted) end is placed in a reservoir full of water, and the other finish placed in a receiving vessel. The reservoir must be higher than the receiving vessel. Due to capillary activeness and gravity, h2o volition slowly transfer from the reservoir to the receiving vessel. This uncomplicated device tin be used to water houseplants when nobody is habitation. This holding is also fabricated utilize of in the lubrication of steam locomotives: wicks of worsted wool are used to describe oil from reservoirs into delivery pipes leading to the bearings.[26]
In plants and animals [edit]
Capillary action is seen in many plants. Water is brought high upwardly in trees past branching; evaporation at the leaves creating depressurization; probably by osmotic pressure added at the roots; and possibly at other locations inside the plant, specially when gathering humidity with air roots.[27] [28]
Capillary action for uptake of h2o has been described in some pocket-sized animals, such as Ligia exotica [29] and Moloch horridus.[30]
Height of a meniscus [edit]
Capillary rise of liquid in a capillary [edit]

Water height in a capillary plotted against capillary bore
The height h of a liquid column is given by Jurin'south law[31]
where is the liquid-air surface tension (forcefulness/unit length), θ is the contact angle, ρ is the density of liquid (mass/volume), g is the local acceleration due to gravity (length/square of time[32]), and r is the radius of tube.
Equally r is in the denominator, the thinner the infinite in which the liquid tin can travel, the farther upward it goes. Too, lighter liquid and lower gravity increment the height of the cavalcade.
For a water-filled drinking glass tube in air at standard laboratory atmospheric condition, γ = 0.0728 Due north/thousand at 20°C, ρ = 1000 kg/m3 , and yard = 9.81 thousand/s2 . Because h2o spreads on clean glass, the constructive equilibrium contact angle is approximately cypher.[33] For these values, the height of the water column is
Thus for a 2 m (6.six ft) radius glass tube in lab atmospheric condition given to a higher place, the water would rising an unnoticeable 0.007 mm (0.00028 in). However, for a 2 cm (0.79 in) radius tube, the water would rise 0.7 mm (0.028 in), and for a 0.2 mm (0.0079 in) radius tube, the water would ascent 70 mm (2.eight in).
Capillary ascent of liquid between two glass plates [edit]
The production of layer thickness (d) and summit height (h) is constant (d·h = constant), the two quantities are inversely proportional. The surface of the liquid between the planes is hyperbola.
- H2o between two glass plates
-

-

-

-

-

-

Liquid transport in porous media [edit]

Capillary catamenia in a brick, with a sorptivity of 5.0 mm·min−i/2 and a porosity of 0.25.
When a dry porous medium is brought into contact with a liquid, it volition blot the liquid at a charge per unit which decreases over fourth dimension. When considering evaporation, liquid penetration will reach a limit dependent on parameters of temperature, humidity and permeability. This process is known equally evaporation express capillary penetration [25] and is widely observed in mutual situations including fluid absorption into paper and rising clammy in concrete or masonry walls. For a bar shaped section of material with cross-exclusive area A that is wetted on one end, the cumulative volume V of absorbed liquid after a time t is
where S is the sorptivity of the medium, in units of m·s−1/2 or mm·min−one/two. This time dependence relation is similar to Washburn'southward equation for the wicking in capillaries and porous media.[34] The quantity
is chosen the cumulative liquid intake, with the dimension of length. The wetted length of the bar, that is the altitude between the wetted end of the bar and the and so-called wet front, is dependent on the fraction f of the volume occupied by voids. This number f is the porosity of the medium; the wetted length is then
Some authors utilise the quantity S/f equally the sorptivity.[35] The in a higher place description is for the case where gravity and evaporation do non play a role.
Sorptivity is a relevant property of edifice materials, considering it affects the corporeality of rising dampness. Some values for the sorptivity of building materials are in the tabular array beneath.
| Material | Sorptivity (mm·min−one/2) |
|---|---|
| Aerated concrete | 0.l |
| Gypsum plaster | 3.l |
| Dirt brick | ane.16 |
| Mortar | 0.70 |
| Concrete brick | 0.xx |
See also [edit]
- Bond number
- Spring water
- Capillary fringe
- Capillary pressure
- Capillary wave
- Capillary bridges
- Clammy-proof grade
- Darcy'south police force
- Frost flowers
- Frost heaving
- Hindu milk miracle
- Krogh model
- Mercury intrusion porosimetry
- Needle ice
- Surface tension
- Washburn's equation
- Water
- Wick effect
- Immature–Laplace equation
References [edit]
- ^ "Capillary Action – Liquid, Water, Strength, and Surface – JRank Articles". Scientific discipline.jrank.org. Archived from the original on 2013-05-27. Retrieved 2013-06-18 .
- ^ Freeman, Scott (2014). Biological Sciences. The states: Pearson. pp. 765–766. ISBN978-0-321-74367-1.
- ^ Simon, E.J., Dickey, J.L, & Reece, J.B. (2019). Campbell essential biology. 7th New York: Pearson
- ^ Run into:
- Manuscripts of Léonardo de Vinci (Paris), vol. North, folios 11, 67, and 74.
- Guillaume Libri, Histoire des sciences mathématiques en Italie, depuis la Renaissance des lettres jusqu'a la fin du dix-septième siecle [History of the mathematical sciences in Italy, from the Renaissance until the cease of the seventeenth century] (Paris, France: Jules Renouard et cie., 1840), vol. three, folio 54 Archived 2016-12-24 at the Wayback Machine. From page 54: "Enfin, deux observations capitales, celle de l'action capillaire (vii) et celle de la diffraction (eight), dont jusqu'à présent on avait méconnu le véritable auteur, sont dues également à ce brillant génie." (Finally, 2 major observations, that of capillary action (7) and that of diffraction (8), the true writer of which until now had non been recognized, are as well due to this brilliant genius.)
- C. Wolf (1857) "Vom Einfluss der Temperatur auf die Erscheinungen in Haarröhrchen" (On the influence of temperature on phenomena in capillary tubes) Annalen der Physik und Chemie, 101 (177) : 550–576 ; meet footnote on folio 551 Archived 2014-06-29 at the Wayback Motorcar by editor Johann C. Poggendorff. From page 551: " ... nach Libri (Hist. des sciences math. en Italie, T. III, p. 54) in den zu Paris aufbewahrten Handschriften des grossen Künstlers Leonardo da Vinci (gestorben 1519) schon Beobachtungen dieser Art vorfinden; ... " ( ... according to Libri (History of the mathematical sciences in Italy, vol. 3, p. 54) observations of this kind [i.e., of capillary action] are already to exist found in the manuscripts of the great artist Leonardo da Vinci (died 1519), which are preserved in Paris; ... )
- ^ More detailed histories of research on capillary action can be constitute in:
- David Brewster, ed., Edinburgh Encyclopaedia (Philadelphia, Pennsylvania: Joseph and Edward Parker, 1832), volume 10, pp. 805–823 Archived 2016-12-24 at the Wayback Machine.
- Maxwell, James Clerk; Strutt, John William (1911). . In Chisholm, Hugh (ed.). Encyclopædia Britannica. Vol. 5 (11th ed.). Cambridge University Press. pp. 256–275.
- John Uri Lloyd (1902) "References to capillarity to the end of the year 1900," Archived 2014-12-14 at the Wayback Machine Bulletin of the Lloyd Library and Museum of Botany, Pharmacy and Materia Medica, 1 (4) : 99–204.
- ^ In his book of 1759, Giovani Batista Clemente Nelli (1725–1793) stated (p. 87) that he had "un libro di problem vari geometrici ec. due east di speculazioni, ed esperienze fisiche ec." (a volume of diverse geometric issues and of speculation and physical experiments, etc.) past Aggiunti. On pages 91–92, he quotes from this book: Aggiunti attributed capillary activeness to "moto occulto" (hidden/secret motion). He proposed that mosquitoes, butterflies, and bees feed via capillary activity, and that sap ascends in plants via capillary action. See: Giovambatista Clemente Nelli, Saggio di Storia Letteraria Fiorentina del Secolo XVII ... [Essay on Florence's literary history in the 17th century, ... ] (Lucca, (Italy): Vincenzo Giuntini, 1759), pp. 91–92. Archived 2014-07-27 at the Wayback Car
- ^ Robert Boyle, New Experiments Physico-Mechanical touching the Bound of the Air, ... (Oxford, England: H. Hall, 1660), pp. 265–270. Available on-line at: Echo (Max Planck Institute for the History of Science; Berlin, Germany) Archived 2014-03-05 at the Wayback Machine.
- ^ Run into, for example:
- Robert Hooke (1661) An endeavour for the explication of the Phenomena appreciable in an experiment published past the Right Hon. Robert Boyle, in the 35th experiment of his Epistolical Discourse touching the Air, in confirmation of a former conjecture fabricated by R. Hooke. [pamphlet].
- Hooke'due south An attempt for the explication ... was reprinted (with some changes) in: Robert Hooke, Micrographia ... (London, England: James Allestry, 1667), pp. 12–22, "Observ. IV. Of small Drinking glass Canes." Archived 2016-12-24 at the Wayback Motorcar
- Geminiano Montanari, Pensieri fisico-matematici sopra alcune esperienze fatte in Bologna ... Archived 2016-12-29 at the Wayback Machine [Physical-mathematical ideas about some experiments done in Bologna ... ] (Bologna, (Italian republic): 1667).
- George Sinclair, Ars Nova et Magna Gravitatis et Levitatis Archived 2017-11-03 at the Wayback Machine [New and peachy powers of weight and levity] (Rotterdam, Netherlands: Arnold Leers, Jr., 1669).
- Johannes Christoph Sturm, Collegium Experimentale sive Curiosum [Catalog of experiments, or Curiosity] (Nüremberg (Norimbergæ), (Germany): Wolfgang Moritz Endter & the heirs of Johann Andreas Endter, 1676). See: "Tentamen 8. Canaliculorum angustiorum recens-notata Phænomena, ... " Archived 2014-06-29 at the Wayback Car (Essay 8. Recently noted phenomena of narrow capillaries, ... ), pp. 44–48.
- ^ Come across:
- Honorato Fabri, Dialogi physici ... ((Lyon (Lugdunum), France: 1665), pages 157 ff Archived 2016-12-24 at the Wayback Machine "Dialogus Quartus. In quo, de libratis suspensisque liquoribus & Mercurio disputatur. (Dialogue 4. In which the balance and suspension of liquids and mercury is discussed).
- Honorato Fabri, Dialogi physici ... ((Lyon (Lugdunum), France: Antoine Molin, 1669), pages 267 ff Archived 2017-04-07 at the Wayback Car "Alithophilus, Dialogus quartus, in quo nonnulla discutiuntur à D. Montanario opposita circa elevationem Humoris in canaliculis, etc." (Alithophilus, Fourth dialogue, in which Dr. Montanari's opposition regarding the elevation of liquids in capillaries is utterly refuted).
- ^ Jacob Bernoulli, Dissertatio de Gravitate Ætheris Archived 2017-04-07 at the Wayback Auto (Amsterdam, Netherlands: Hendrik Wetsten, 1683).
- ^ Isaac Vossius, De Nili et Aliorum Fluminum Origine [On the sources of the Nile and other rivers] (Hague (Hagæ Comitis), Netherlands: Adrian Vlacq, 1666), pages 3–7 Archived 2017-04-07 at the Wayback Machine (chapter 2).
- ^ Borelli, Giovanni Alfonso De motionibus naturalibus a gravitate pendentibus (Lyon, French republic: 1670), folio 385, Cap. 8 Prop. CLXXXV (Chapter 8, Proffer 185.). Bachelor on-line at: Echo (Max Planck Institute for the History of Scientific discipline; Berlin, Germany) Archived 2016-12-23 at the Wayback Machine.
- ^ Carré (1705) "Experiences sur les tuyaux Capillaires" Archived 2017-04-07 at the Wayback Automobile (Experiments on capillary tubes), Mémoires de l'Académie Royale des Sciences, pp. 241–254.
- ^ Encounter:
- Francis Hauksbee (1708) "Several Experiments Touching the Seeming Spontaneous Ascent of Water," Archived 2014-06-29 at the Wayback Machine Philosophical Transactions of the Royal Order of London, 26 : 258–266.
- Francis Hauksbee, Physico-mechanical Experiments on Various Subjects ... (London, England: (Cocky-published), 1709), pages 139–169.
- Francis Hauksbee (1711) "An account of an experiment touching the direction of a drib of oil of oranges, between two glass planes, towards whatsoever side of them that is nearest printing'd together," Philosophical Transactions of the Majestic Society of London, 27 : 374–375.
- Francis Hauksbee (1712) "An account of an experiment touching the ascent of h2o betwixt two glass planes, in an hyperbolick effigy," Philosophical Transactions of the Royal Lodge of London, 27 : 539–540.
- ^ See:
- Josia Weitbrecht (1736) "Tentamen theoriae qua ascensus aquae in tubis capillaribus explicatur" Archived 2014-06-29 at the Wayback Machine (Theoretical essay in which the ascent of water in capillary tubes is explained), Commentarii academiae scientiarum imperialis Petropolitanae (Memoirs of the imperial academy of sciences in St. Petersburg), 8 : 261–309.
- Josia Weitbrecht (1737) "Explicatio difficilium experimentorum circa ascensum aquae in tubis capillaribus" Archived 2014-11-05 at the Wayback Machine (Explanation of difficult experiments concerning the ascent of water in capillary tubes), Commentarii academiae scientiarum imperialis Petropolitanae (Memoirs of the imperial university of sciences in St. Petersburg), 9 : 275–309.
- ^ For instance:
- In 1740, Christlieb Ehregott Gellert (1713–1795) observed that like mercury, molten lead would not adhere to drinking glass and therefore the level of molten atomic number 82 was depressed in a capillary tube. See: C. E. Gellert (1740) "De phenomenis plumbi fusi in tubis capillaribus" (On phenomena of molten lead in capillary tubes) Commentarii academiae scientiarum imperialis Petropolitanae (Memoirs of the royal academy of sciences in St. Petersburg), 12 : 243–251. Bachelor on-line at: Annal.org Archived 2016-03-17 at the Wayback Machine.
- Gaspard Monge (1746–1818) investigated the force between panes of drinking glass that were separated past a moving picture of liquid. Run across: Gaspard Monge (1787) "Mémoire sur quelques effets d'allure ou de répulsion apparente entre les molécules de matière" Archived 2016-03-16 at the Wayback Machine (Memoir on some furnishings of the apparent attraction or repulsion between molecules of matter), Histoire de l'Académie royale des sciences, avec les Mémoires de l'Académie Royale des Sciences de Paris (History of the Royal University of Sciences, with the Memoirs of the Royal University of Sciences of Paris), pp. 506–529. Monge proposed that particles of a liquid exert, on each other, a brusque-range strength of attraction, and that this strength produces the surface tension of the liquid. From p. 529: "En supposant ainsi que l'adhérence des molécules d'un liquide n'ait d'effet sensible qu'à la surface même, & dans le sens de la surface, il seroit facile de déterminer la courbure des surfaces des liquides dans le voisinage des parois qui les conteinnent ; ces surfaces seroient des lintéaires dont la tension, constante dans tous les sens, seroit par-tout égale à l'adhérence de deux molécules ; & les phénomènes des tubes capillaires n'auroient plus rein qui ne pût être déterminé par l'analyse." (Thus by bold that the adhesion of a liquid's molecules has a significant effect only at the surface itself, and in the direction of the surface, it would be piece of cake to determine the curvature of the surfaces of liquids in the vicinity of the walls that contain them ; these surfaces would exist menisci whose tension, [being] constant in every management, would be everywhere equal to the adhesion of two molecules ; and the phenomena of capillary tubes would have aught that could not be determined by analysis [i.e., calculus].)
- ^ In the 18th century, some investigators did attempt a quantitative treatment of capillary action. Run into, for example, Alexis Claude Clairaut (1713–1765) Theorie de la Figure de la Terre tirée des Principes de l'Hydrostatique [Theory of the figure of the Earth based on principles of hydrostatics] (Paris, France: David fils, 1743), Chapitre X. De l'élevation ou de 50'abaissement des Liqueurs dans les Tuyaux capillaires (Chapter ten. On the summit or depression of liquids in capillary tubes), pages 105–128. Archived 2016-04-09 at the Wayback Machine
- ^ Thomas Young (January 1, 1805) "An essay on the cohesion of fluids," Archived 2014-06-30 at the Wayback Automobile Philosophical Transactions of the Purple Gild of London, 95 : 65–87.
- ^ Pierre Simon marquis de Laplace, Traité de Mécanique Céleste, volume 4, (Paris, France: Courcier, 1805), Supplément au dixième livre du Traité de Mécanique Céleste, pages 1–79 Archived 2016-12-24 at the Wayback Automobile.
- ^ Carl Friedrich Gauss, Principia generalia Theoriae Figurae Fluidorum in statu Aequilibrii [Full general principles of the theory of fluid shapes in a state of equilibrium] (Göttingen, (Germany): Dieterichs, 1830). Available on-line at: Hathi Trust.
- ^ William Thomson (1871) "On the equilibrium of vapour at a curved surface of liquid," Archived 2014-10-26 at the Wayback Machine Philosophical Magazine, series 4, 42 (282) : 448–452.
- ^ Franz Neumann with A. Wangerin, ed., Vorlesungen über die Theorie der Capillarität [Lectures on the theory of capillarity] (Leipzig, Frg: B. One thousand. Teubner, 1894).
- ^ Albert Einstein (1901) "Folgerungen aus den Capillaritätserscheinungen" Archived 2017-10-25 at the Wayback Machine (Conclusions [drawn] from capillary phenomena), Annalen der Physik, 309 (iii) : 513–523.
- ^ Hans-Josef Kuepper. "Listing of Scientific Publications of Albert Einstein". Einstein-website.de. Archived from the original on 2013-05-08. Retrieved 2013-06-xviii .
- ^ a b c Liu, Mingchao; Wu, Jian; Gan, Yixiang; Hanaor, Dorian A.H.; Chen, C.Q. (2018). "Tuning capillary penetration in porous media: Combining geometrical and evaporation effects" (PDF). International Journal of Oestrus and Mass Transfer. 123: 239–250. doi:x.1016/j.ijheatmasstransfer.2018.02.101.
- ^ Ahrons, Ernest Leopold (1922). Lubrication of Locomotives. London: Locomotive Publishing Visitor. p. 26. OCLC 795781750.
- ^ Tree physics Archived 2013-11-28 at the Wayback Machine at "Not bad, Plausible And" scientific discussion website.
- ^ Water in Redwood and other trees, mostly by evaporation Archived 2012-01-29 at the Wayback Machine article at wonderquest website.
- ^ Ishii D, Horiguchi H, Hirai Y, Yabu H, Matsuo Y, Ijiro K, Tsujii K, Shimozawa T, Hariyama T, Shimomura Thou (October 23, 2013). "H2o send mechanism through open capillaries analyzed past direct surface modifications on biological surfaces". Scientific Reports. 3: 3024. Bibcode:2013NatSR...3E3024I. doi:10.1038/srep03024. PMC3805968. PMID 24149467.
- ^ Bentley PJ, Blumer WF (1962). "Uptake of water by the lizard, Moloch horridus". Nature. 194 (4829): 699–670 (1962). Bibcode:1962Natur.194..699B. doi:10.1038/194699a0. PMID 13867381. S2CID 4289732.
- ^ G.Thou. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Printing (1967) ISBN 0-521-66396-2,
- ^ Hsai-Yang Fang, john L. Daniels, Introductory Geotechnical Engineering: An Environmental Perspective
- ^ "Capillary Tubes - an overview | ScienceDirect Topics". www.sciencedirect.com . Retrieved 2021-10-29 .
- ^ Liu, M.; et al. (2016). "Evaporation limited radial capillary penetration in porous media" (PDF). Langmuir. 32 (38): 9899–9904. doi:x.1021/acs.langmuir.6b02404. PMID 27583455.
- ^ C. Hall, Due west.D. Hoff, Water send in brick, stone, and concrete. (2002) page 131 on Google books Archived 2014-02-20 at the Wayback Machine
- ^ Hall and Hoff, p. 122
Farther reading [edit]
- de Gennes, Pierre-Gilles; Brochard-Wyart, Françoise; Quéré, David (2004). Capillarity and Wetting Phenomena. Springer New York. doi:ten.1007/978-0-387-21656-0. ISBN978-i-4419-1833-8.
Source: https://en.wikipedia.org/wiki/Capillary_action
Posted by: urbanekunked1956.blogspot.com








0 Response to "What Does Capillary Action Do For Plants And Animals"
Post a Comment